Congratulations to Filipa Sousa and Val Karavaeva for their contribution to the new publication in Froniers in Genetics:
"In Campylobacter jejuni, a new type of chaperone receives heme from ferrochelatase"
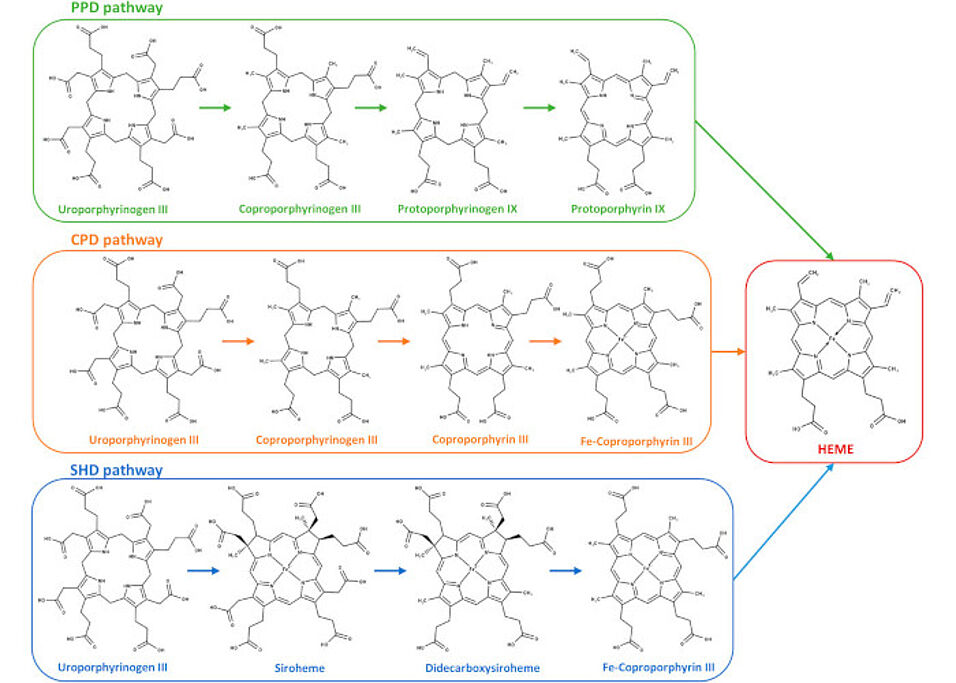
In this study, C. jejuni CgdH2 heme-binding protein was discovered and functionally characterized by kinetic and mutagenesis assays. CgdH2 was shown to interact with ferrochelatase suggesting its role in facilitating heme transfer from ferrochelatase to CgdH2 in the PPD pathway. Phylogenetic analysis demonstrated that CgdH2 in C. jejuni is evolutionarily distinct from known chaperones. Consequently, CgdH2 is the first protein identified as an acceptor of intracellularly formed heme, significantly advancing our understanding of heme trafficking mechanisms within bacterial cells.
Original article:
Zamarreño Beas, J., Videira, M. A., Karavaeva, V., Lourenço, F. M., Almeida, M. R., Sousa, F., & Saraiva, L. M. (2023). In Campylobacter jejuni, a new type of chaperone receives heme from ferrochelatase. Frontiers in Genetics, 14, 1199357.